Therapeutic Targeting of Neutrophil Netosis and Phagocytosis
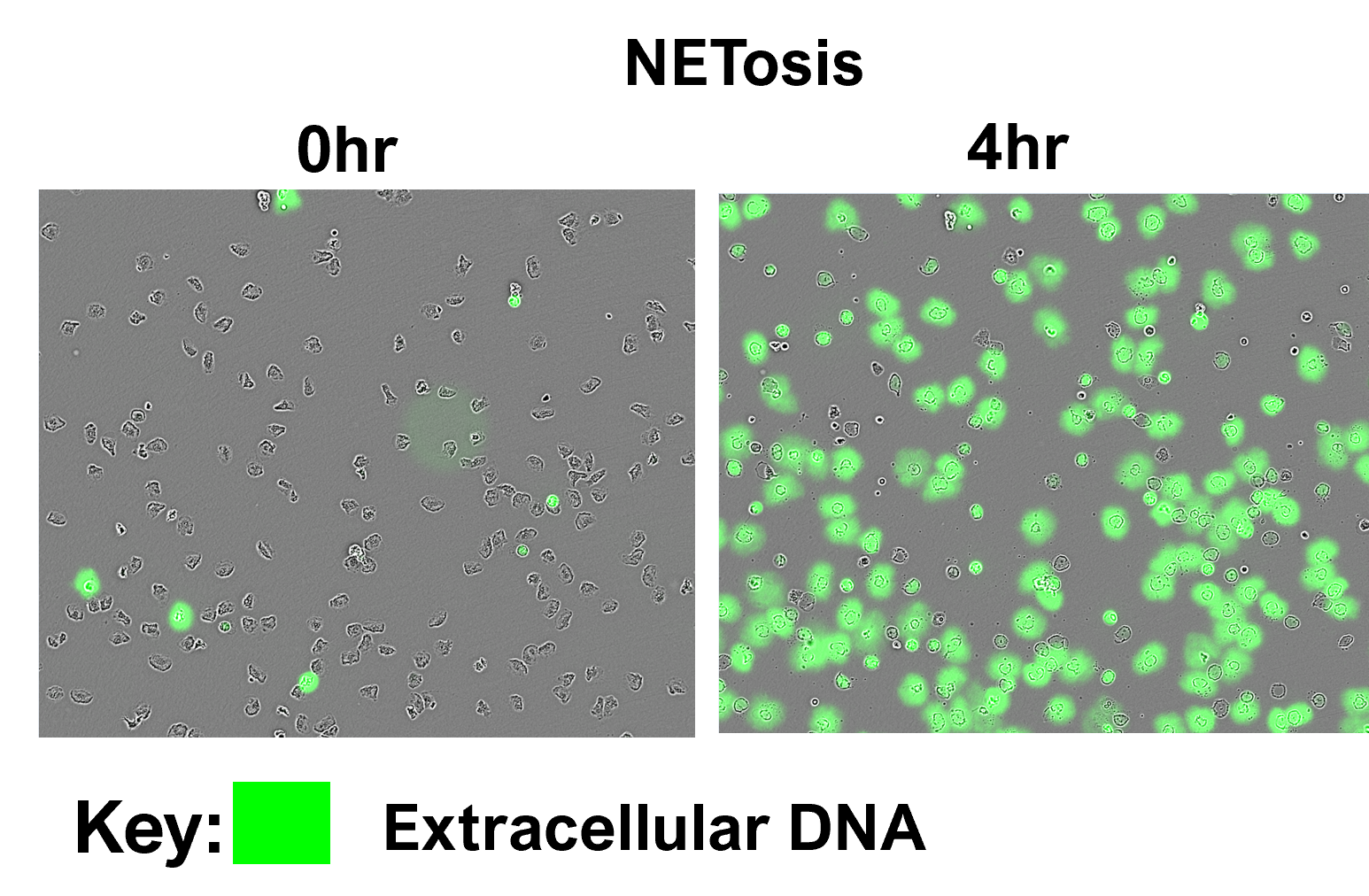
Neutrophils are amongst the first immune cells to respond to injury or infection. Armed with a variety of effector mechanisms, they release neutrophil extracellular traps (NETs), comprising of chromatin and antimicrobial proteins which are released via a unique, pro-inflammatory form of cell death called NETosis. Dysregulated NET release can damage the host, contributing to autoimmune diseases such as Systemic Lupus Erythematosus (SLE) by the release of autoantigens. Similarly, dysregulated NET release contributes to diseases such as atherosclerosis, deep vein thrombosis and has been shown to promote cancer progression and metastasis. Modulating neutrophil function is therefore a potential therapeutic intervention. We present data from NETosis and phagocytosis assays which can be used to determine how a therapeutic alters neutrophil function.
Aim to block netosis to reduce inflammation
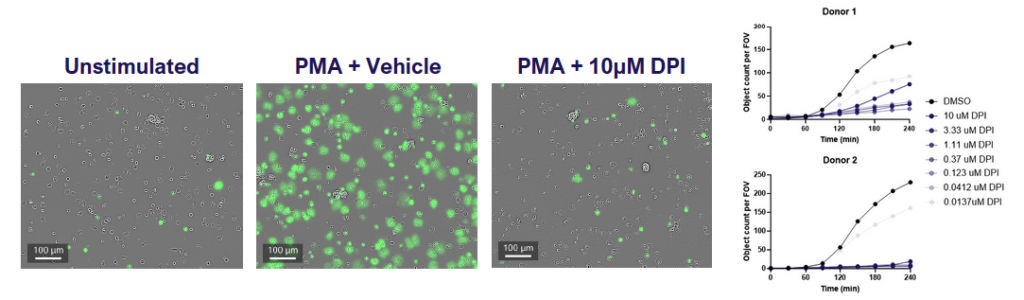
Fig 1: Neutrophils were isolated from fresh blood, incubated with the NOX2 inhibitor (DPI) and then stimulated with PMA in the presence of SYTOX green to detect NETs. Cells were imaged for green fluorescence (NETs) every 30 minutes for 4 hours. Data shows that NETosis increases over time and is inhibited by DPI.
Aim to enhance neutrophil phagocytosis of targets
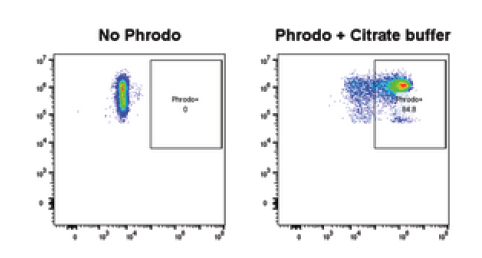
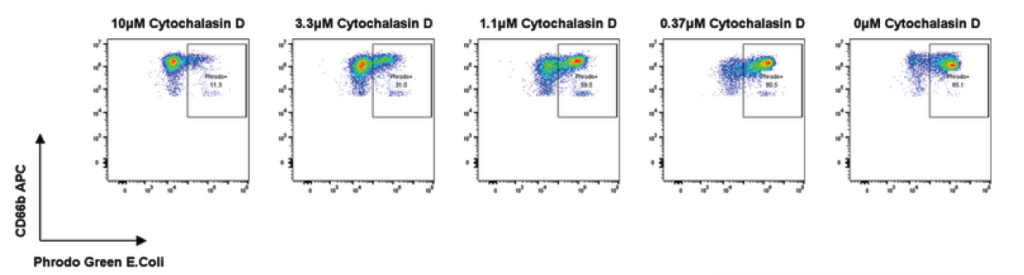
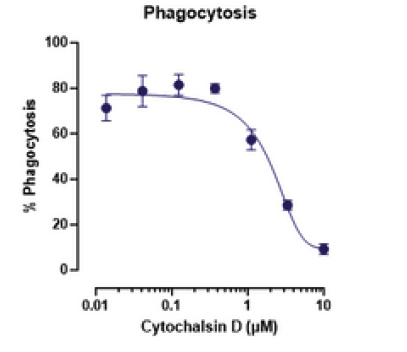
Fig 2: Neutrophils were isolated from fresh blood and incubated with pHrodo-labelled E. coli for 60 minutes to allow phagocytosis to occur. Cells were then stained with CD66b and analysed by flow cytometry; phagocytosing neutrophils were identified as CD66b+ pHrodo+ cells. Citrate buffer was used as a positive control for pHrodo staining. Cytochalasin D is included as a reference inhibitor to demonstrate a dose responsive inhibition of phagocytosis.