3D Tumour Killing Models: Tools for screening immune or tumour targeted therapeutics
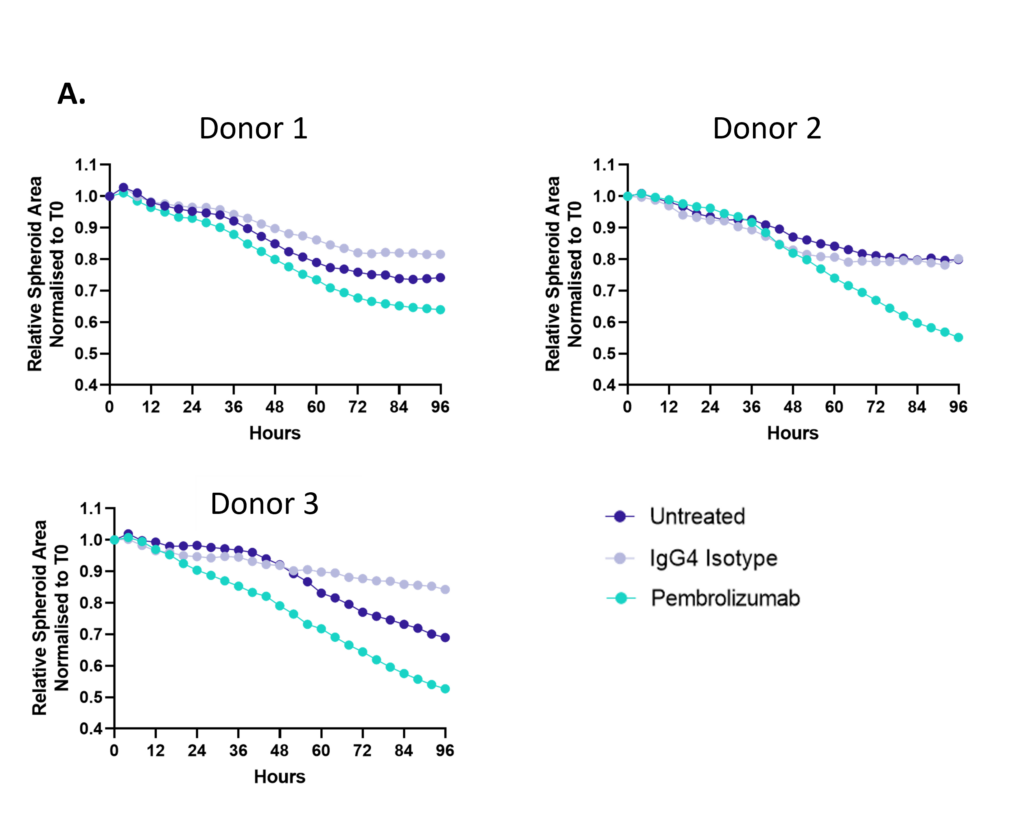
Figure 1: SKOV-3 NLR tumour cells were seeded into 96 well plates, PBMC were added once spheroids were established in the presence of Pembrolizumab or IgG4 control or vehicle (untreated) and imaged every 4 hours for 96 hours using the CellCyte X. Relative spheroid area (%) was measured (A). AUC statistics were calculated using GraphPad Prism v9.5.0
Enzyme-Linked Immuno Spot (ELISpot) is a technique which quantifies immune cells of low abundance which release biomarkers such as cytokines in response to antigenic stimulation. ELISpot is a highly sensitive method to test immune modulators, novel vaccine candidates or de-risk immunogenicity testing in an antigen-specific CD4 and/or CD8 T cell assay.
| Condition | Aim |
| No Stimulation | Negative control |
| PMA | Positive control |
| CERI (CMV, EBV, RSV, Influenza) | MHC-I restricted peptide pool to evaluate modulation of CD8+ T-cell memory response |
| CPI (CMV, Parainfluenza, Influenza) | Positive protein antigens to evaluate modulation of CD4+ T-cell memory response |
| CEF (CMV, EBV, Influenza) | MHC-I restricted peptide pool to evaluate modulation of CD8+ T-cell memory response |
| Cyclosporin A (CsA) | Inhibition of immune response |
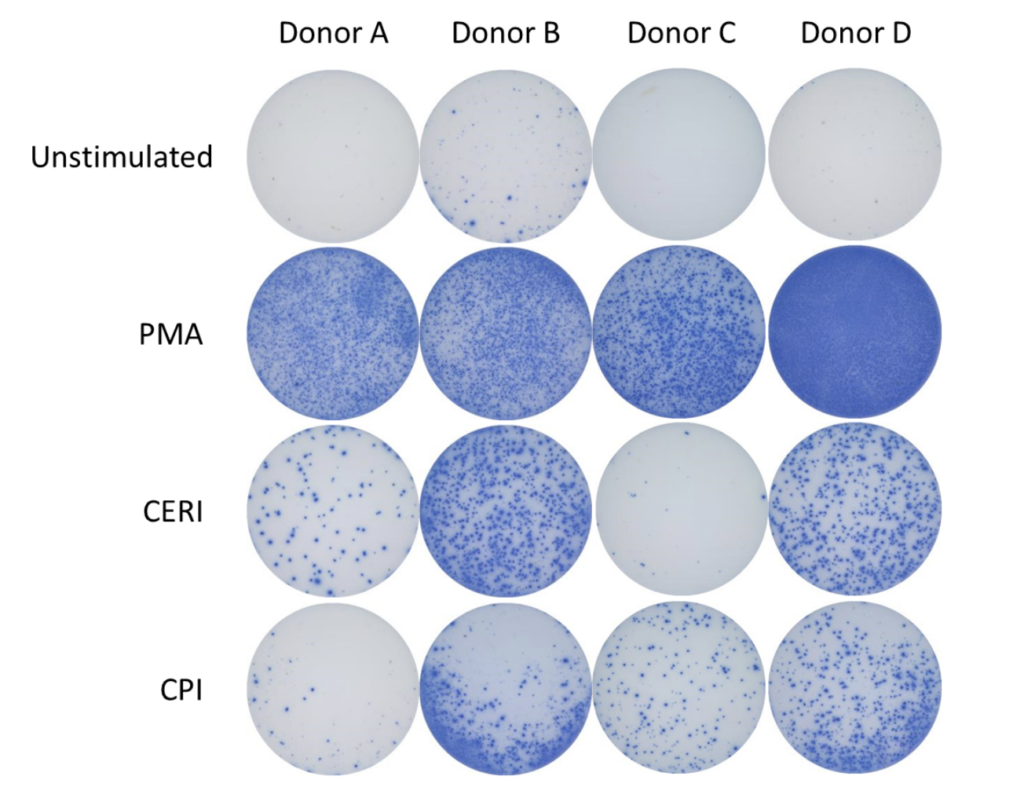
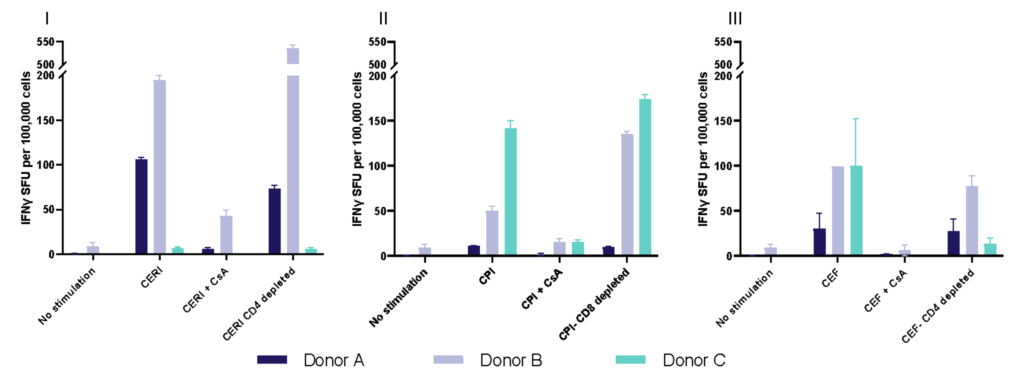
Spot Forming Units (SPU) for IFN-γ per 100,000 PBMC from CERI, CPI and CEF antigens for three donors
Evaluation of therapeutic modulation of antigen-specific memory T cell responses to recall antigens
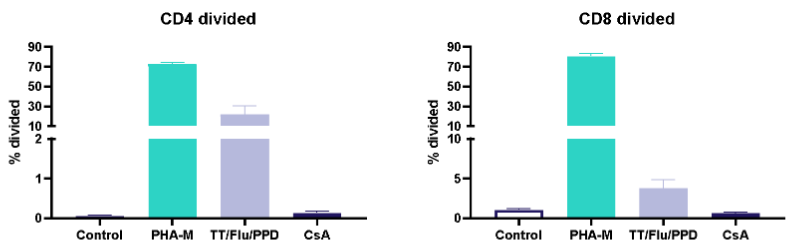
I. T cell response to Tetanus Toxoid, Influenza and PPD antigens
II. Dose response to Influenza antigen
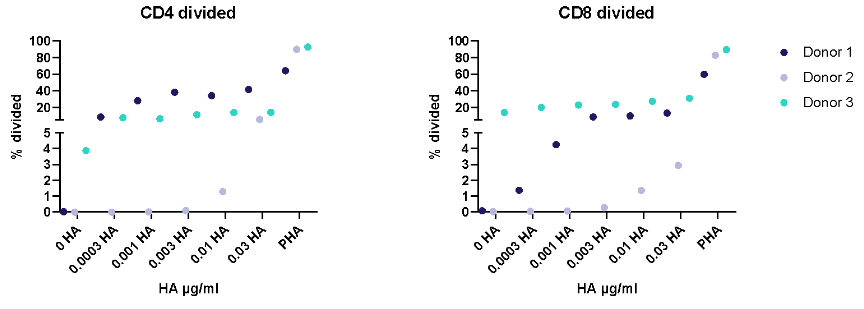
Antigen specific T cells response to a recall antigens. (I) Healthy donors PBMC were stimulated with PHA-M or triple antigen cocktail (Tetanus Toxoid, Influenza and PPD). Cyclosporin was used as a reference treatment. (II) Dose response to Influenza antigen. CD4 and CD8 T cells proliferation was measured by flow cytometry using CTV dilutions.
Dendritic cells fed with tumour cell lysates prime rare neoantigen-specific CD4 T cells and cross present antigen to prime neoantigen-specific naive CD8 T cells
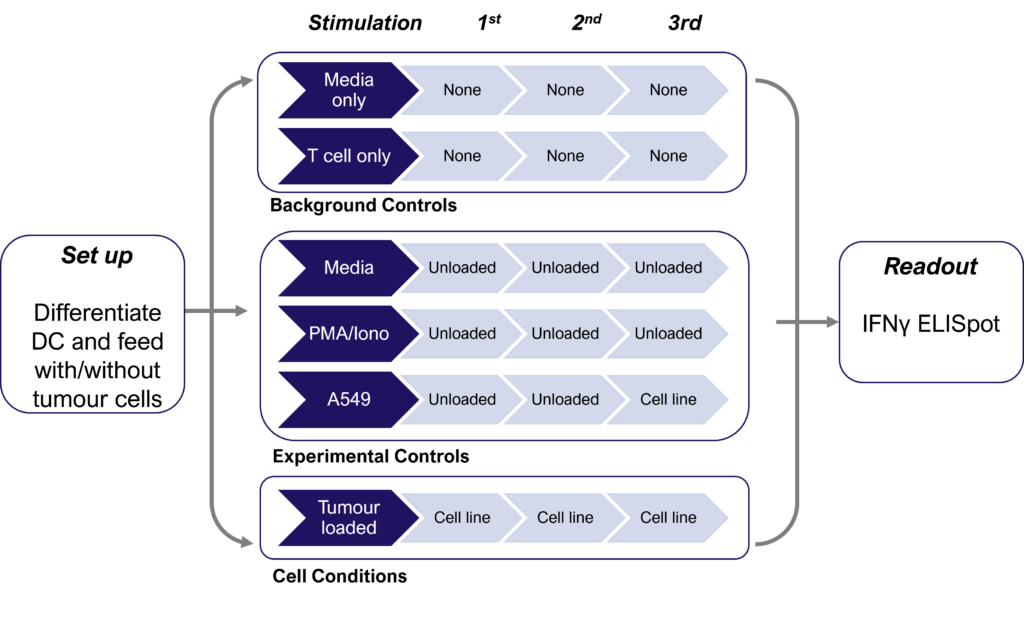
Therapies targeting the modification of tumor cells to increase neoantigen expression and improve immune cell recognition are currently under development. Detecting these subtle changes typically involves several cycles of dendritic cell restimulation of T cells, followed by the sensitive measurement of rare neoantigen-specific T cell responses using IFNγ ELISPOT assays. The schematic below shows the assay design with multiple round of dendritic cell (DC) restimulation to expand rare neoantigen T cell responses.
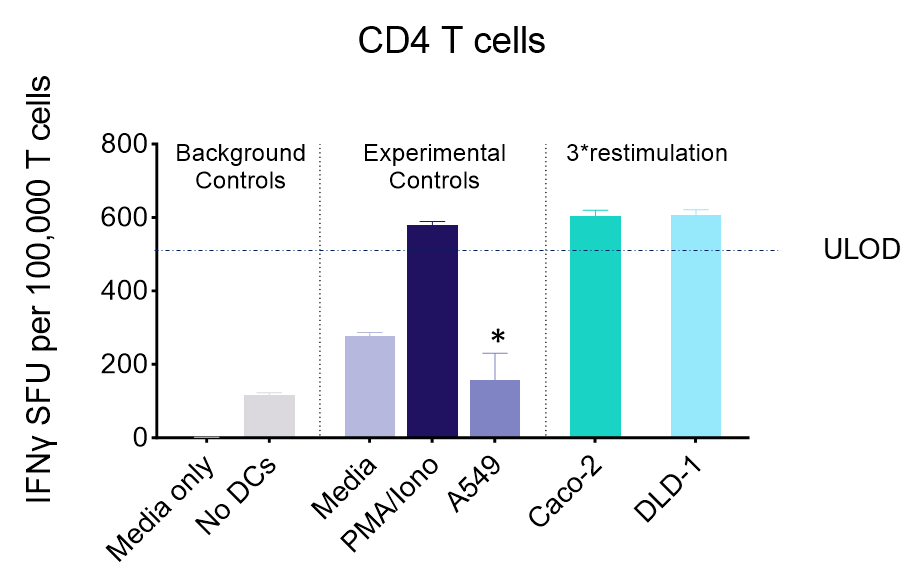
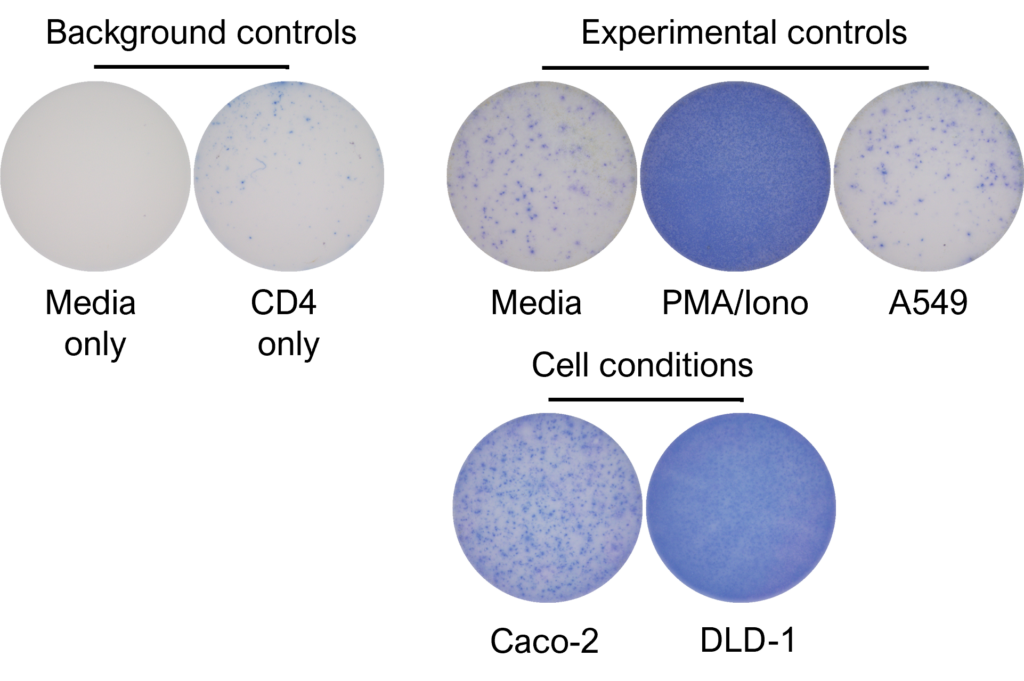
IFNγ release from activated tumour neoantigen-specific CD4 T cells
IFNγ release from activated tumour neoantigen-specific naive CD8 T cells
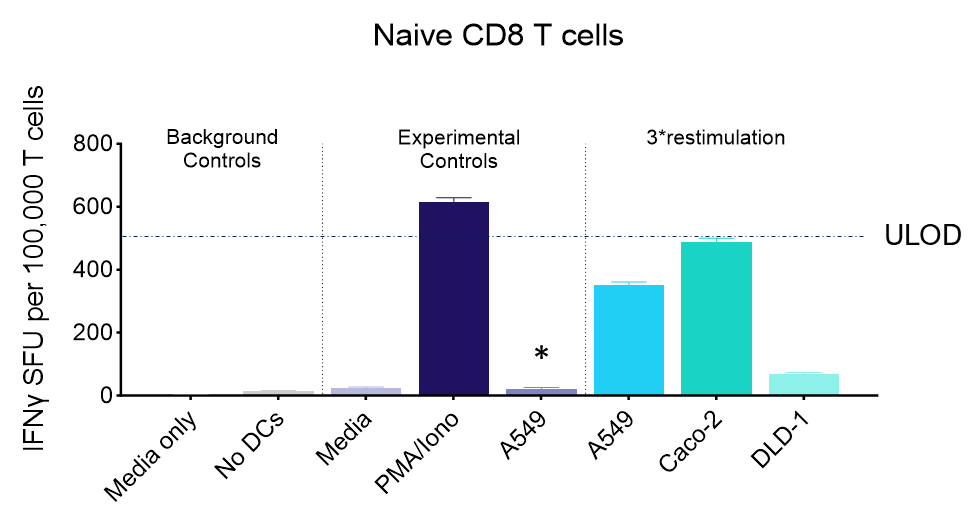
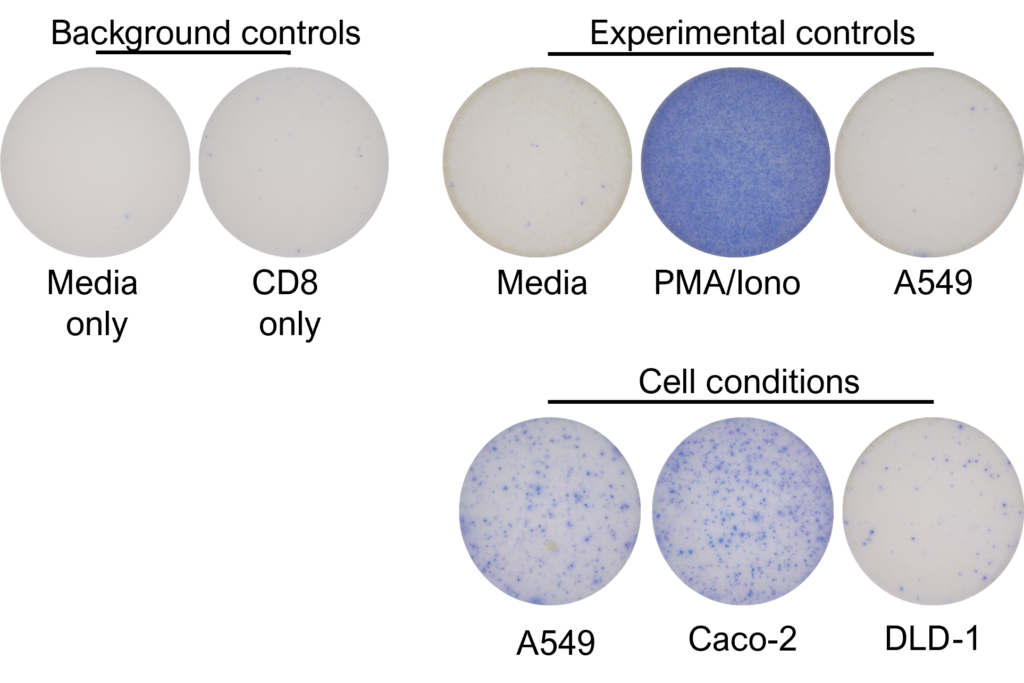
Figures: CD4 or naïve CD8 T cells were co-cultured with unloaded dendritic cells (DC), or with DC loaded with tumour cells that had been previously treated with media or vehicle. Three rounds of stimulation of T cells with DC were performed (* single round of DC stimulation). PMA and ionomycin were added as positive controls during the final round of stimulation. T cell stimulation was assessed by measuring the number of spot forming units (SFU) by IFNγ ELISpot. Media and T cell only wells were included as background controls. Number of SFU per 100,000 plated T cells, with mean of two (experimental controls) or three (all others) replicates ± SEM shown. Representative images of CD4 and naïve CD8 T cell IFNγELISpot. Each blue dot represents one IFNγ-producing T cell. ULOD = Upper Limit of Detection
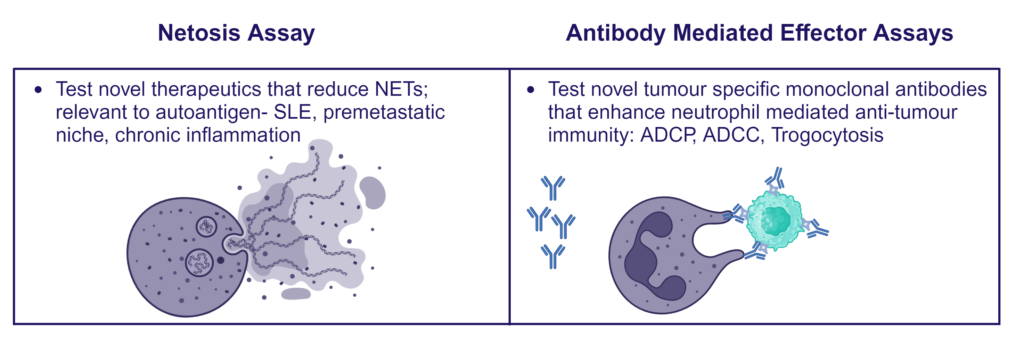
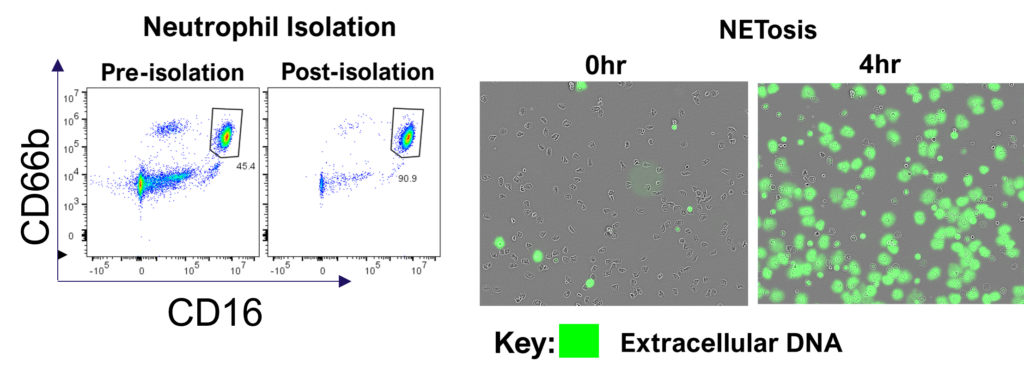
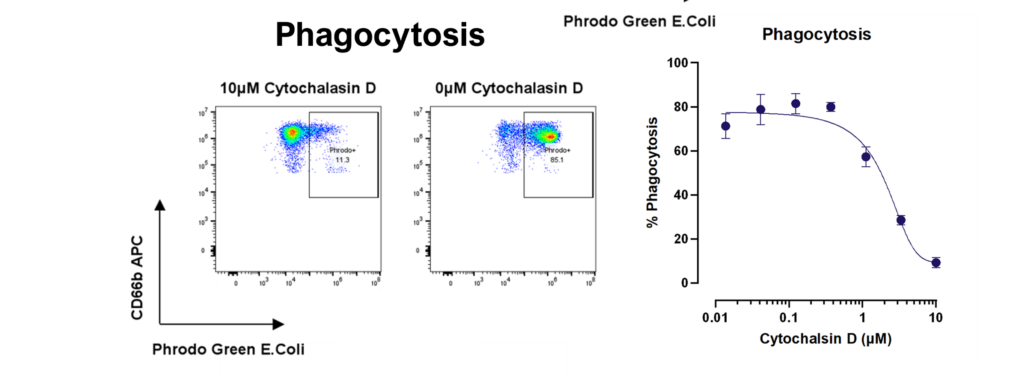
Therapeutic blocking of NETosis and enhancement of neutrophil phagocytosis/trogocytosis
Neutrophils are armed with a variety of effector mechanisms, they release neutrophil extracellular traps (NETs), comprising of chromatin and antimicrobial proteins which are released via a unique, pro-inflammatory form of cell death called NETosis. Dysregulated NET release can damage the host, contributing to autoimmune diseases such as Systemic Lupus Erythematosus (SLE) by the release of autoantigens. Similarly, dysregulated NET release contributes to diseases such as atherosclerosis, deep vein thrombosis and has been shown to promote cancer progression and metastasis. Modulating neutrophil function is therefore a potential therapeutic intervention.
3D Tumour Killing Models: Tools for screening immune or tumour targeted therapeutics


Figure 1: SKOV-3 NLR tumour cells were seeded into 96 well plates, PBMC were added once spheroids were established in the presence of Pembrolizumab or IgG4 control or vehicle (untreated) and imaged every 4 hours for 96 hours using the CellCyte X. Relative spheroid area (%) was measured (A). AUC statistics were calculated using GraphPad Prism v9.5.0
Antibody dependent cell cytotoxicity (ADCC) in a 3D tumour spheroid model
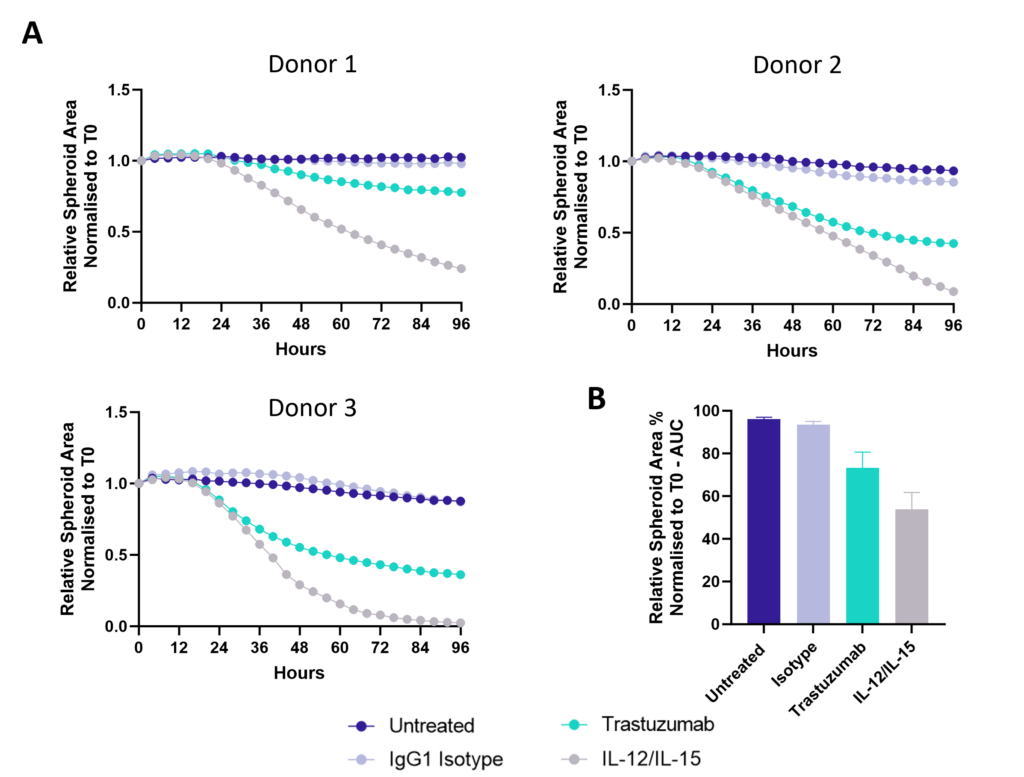
Figure 1: SK-OV-3 NLR cells expressing HER2 were plated were seeded into 96 well plates and once spheroids were established purified NK cells were added to cultures in the presence of Trastuzumab or IgG1 Isotype or IL-12/IL-15 or vehicle (untreated) and imaged every 4 hours for 96 hours using the CellCyte X. Relative spheroid area (%) was measured (A) and the AUC (B) calculated using GraphPad Prism v9.5.0. Line graphs show the mean of triplicate wells and the bar graph shows mean +/- SEM of 3 donors. (C) Representative images from the CellCyte X at 96 hours post NK cell addition to tumour spheroids.
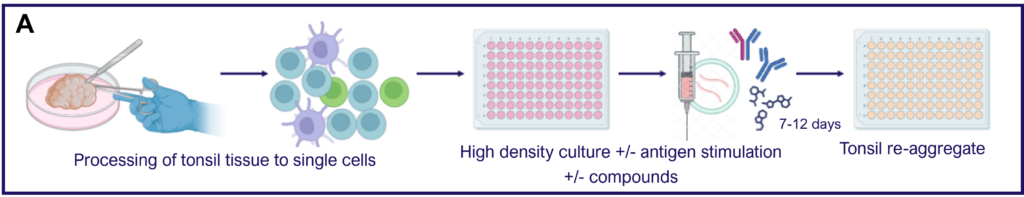
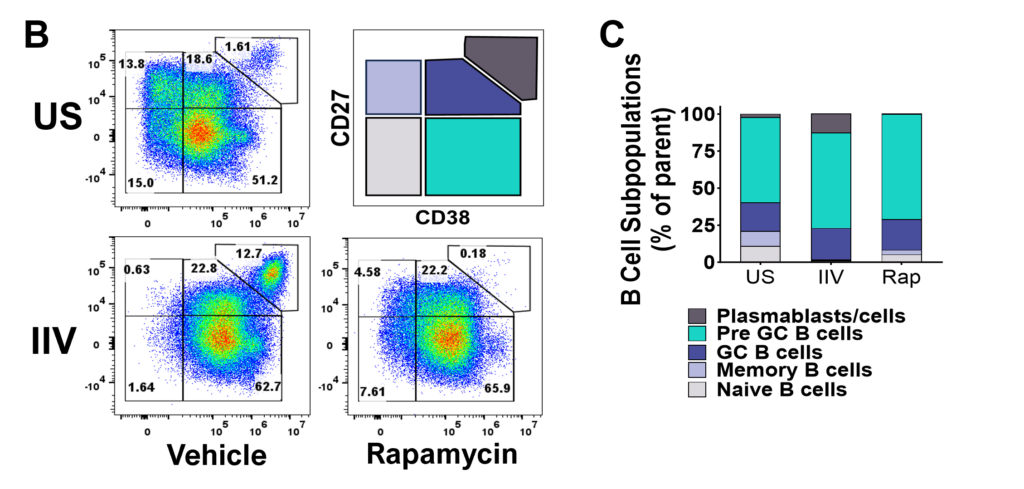
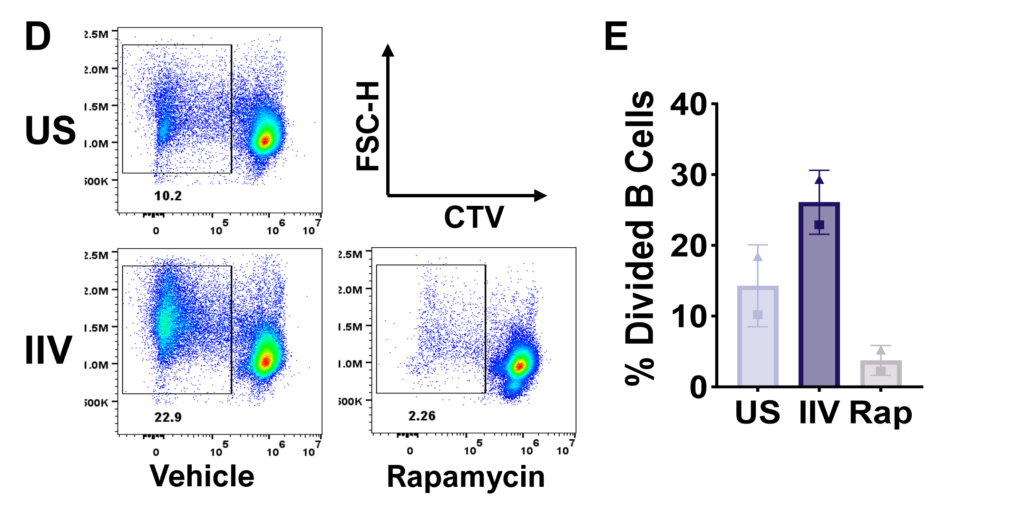
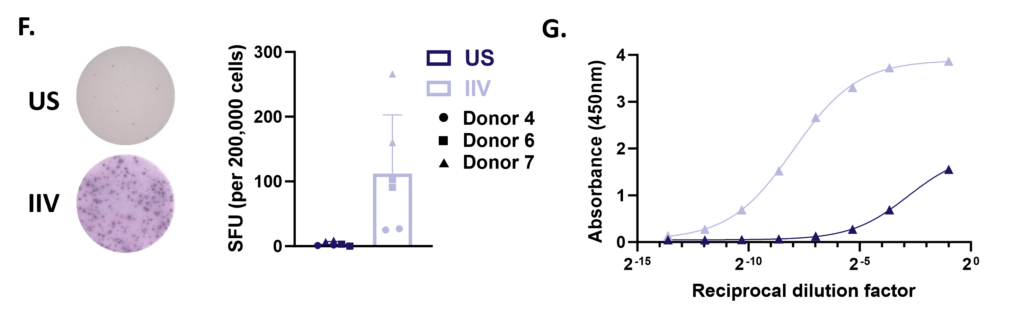
Tonsillar re-aggregate model
Tonsils are a secondary lymphoid tissue rich in B cell subsets and the specialized, anatomically restricted T follicular helper (Tfh) subset of CD4 T cells. Tonsils provide an accessible source of cells for those interested in B cell immunology, Tfh biology and vaccine development. The tonsillar cell re-aggregate model provides functional insight into human germinal center biology, allowing measurement of key features including naive B cell differentiation, plasma cell/plasmablast differentiation, and antibody production.