Therapeutic targeting of CD4+ T cells for Immuno-Oncology and Autoimmunity
Enzyme-Linked Immuno Spot (ELISpot) enables quantification of rare immune cell function in response to antigenic stimulation
ELISpot is a highly sensitive method to test immune modulators, novel vaccine candidates or de-risk immunogenicity testing in an antigen-specific CD4 and/or CD8 T cell assay.
| Condition | Aim |
| No Stimulation | Negative control |
| PMA | Positive control |
| CERI (CMV, EBV, RSV, Influenza) | MHC-I restricted peptide pool to evaluate modulation of CD8+ T-cell memory response |
| CPI (CMV, Parainfluenza, Influenza) | Positive protein antigens to evaluate modulation of CD4+ T-cell memory response |
| CEF (CMV, EBV, Influenza) | MHC-I restricted peptide pool to evaluate modulation of CD8+ T-cell memory response |
| Cyclosporin A (CsA) | Inhibition of immune response |
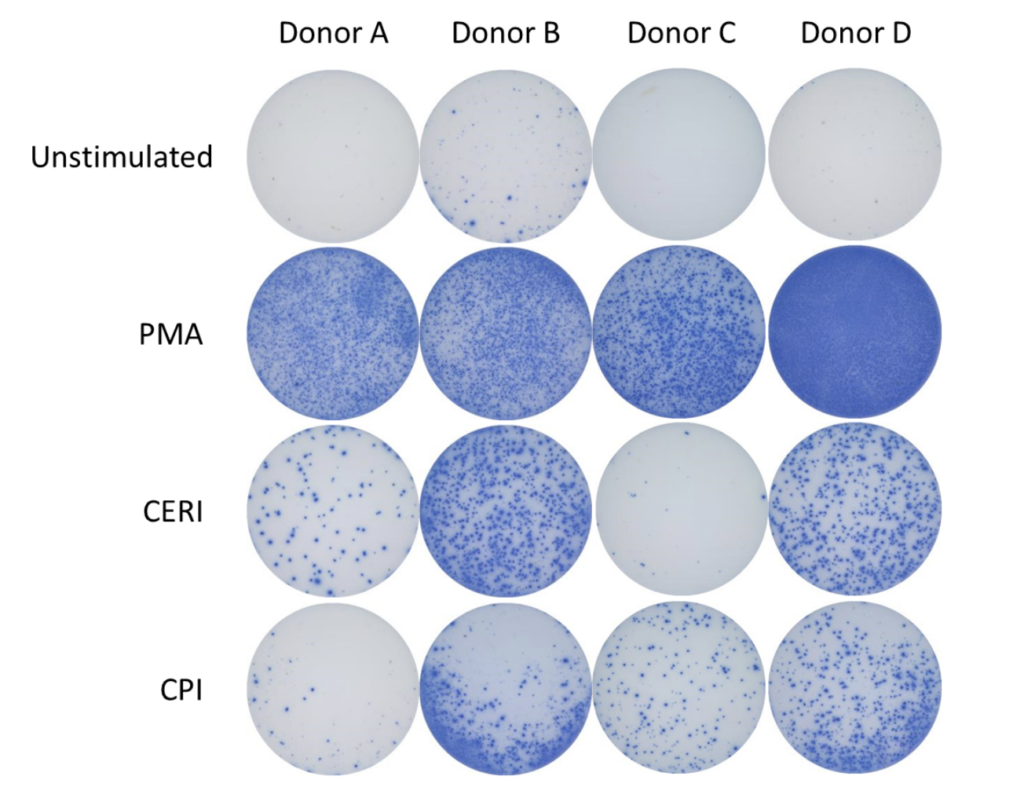
Spot Forming Units (SPU) for IFN-γ per 100,000 PBMC from CERI, CPI and CEF antigens for three donors
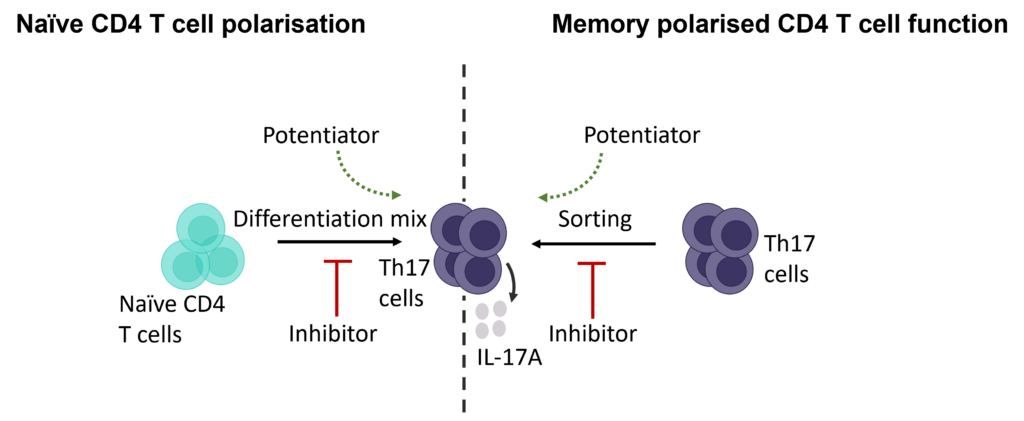
Therapeutic modulation of TH17 CD4+ T cell differentiation or effector function
T helper 17 (Th17) cells, are a subset of CD4 T cells distinguished by their production of IL-17. While crucial for protecting the body against extracellular pathogens, Th17 cells have also been implicated in autoimmune disease. Discovering therapies that target either the differentiation or effector function of Th17 cells could therefore prove beneficial for the treatment of some autoimmune diseases.
Evaluation of therapeutic modulation of Th17 differentiation
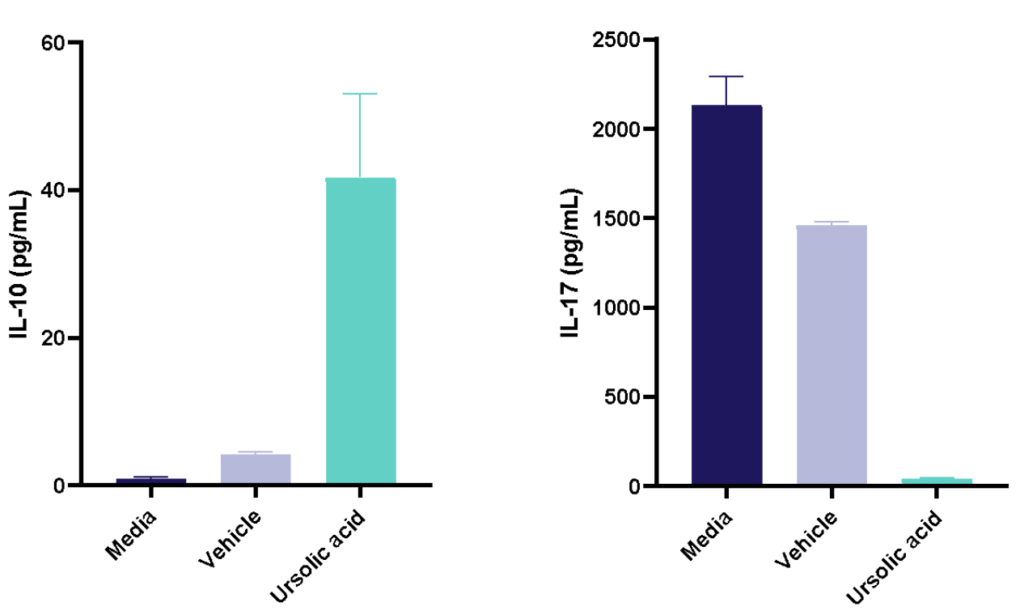
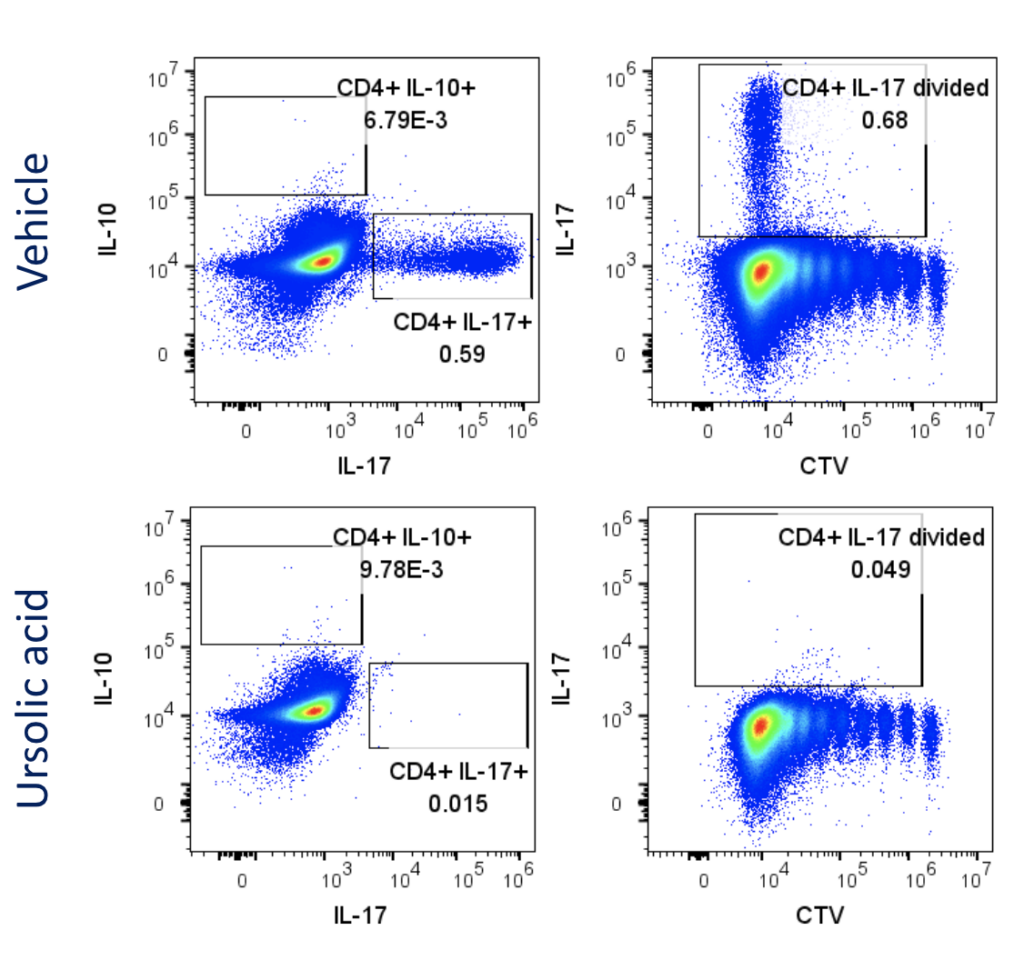
Figure 1: Polarisation of Th17 cells. Naïve CD4 cells were cultured under Th17 polarising conditions for 12 days in the presence or absence of Ursolic Acid. CD4 T cells were assessed for proliferation by CTV dilution; intracellular cytokine staining (ICS) of IL-17 and IL-10 by flow cytometry. On Day 12, Supernatants were collected and evaluated for IL-17 and IL-10 levels by MSD. RoRγT inhibitor Ursolic Acid showed selective inhibition of IL-17 production by intracellular cytokine staining (ICS) and MSD.
Evaluation of therapeutic modulation of Th17 effector function
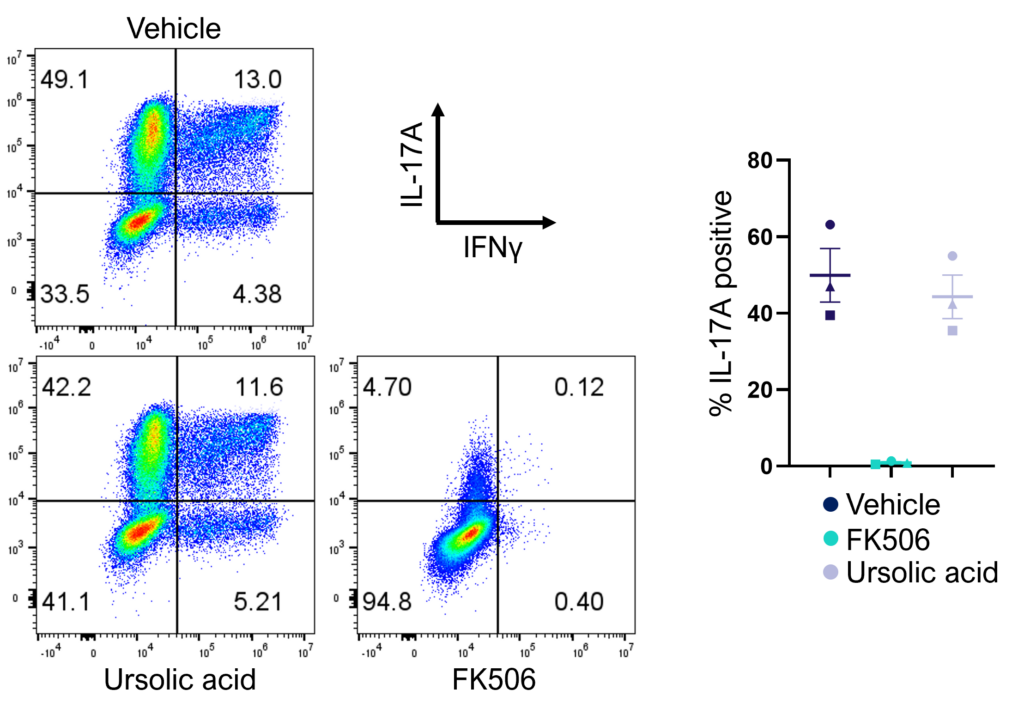
Figure 2: Inhibition of Th17 cell effector function. Magnetically sorted memory Th17 cells were polyclonally stimulated under Th17 conditions in the presence or absence of ursolic acid or FK506 for 5 days. A percentage of Th17 cells are polyfunctional (IL- 17A+IFNγ+) and refractory to inhibition by ursolic acid (an inhibitor of Th17 differentiation) but not FK506.
Therapeutic modulation of DC, CD4+, CD8+ T cell effector function
For many the humble “workhorse” mixed lymphocyte reaction (MLR) is a key screening assay for immuno-therapeutics. 1-way (DC and T cells) and 2-way MLRs (PBMC) provide expression of key ligands on human immune cells for therapeutic modulation.
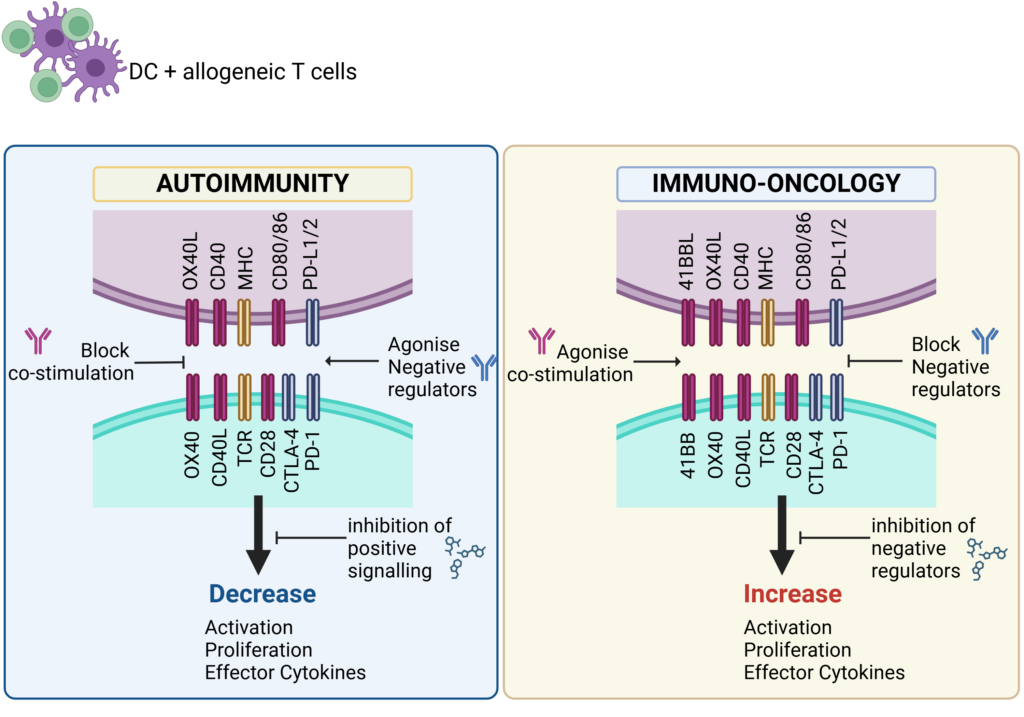
Schematic showing key therapeutic targets that are blocked (antagonise) or engaged (agonise) to control immune responses in Autoimmunity or boost immune responses for Immuno-Oncology.
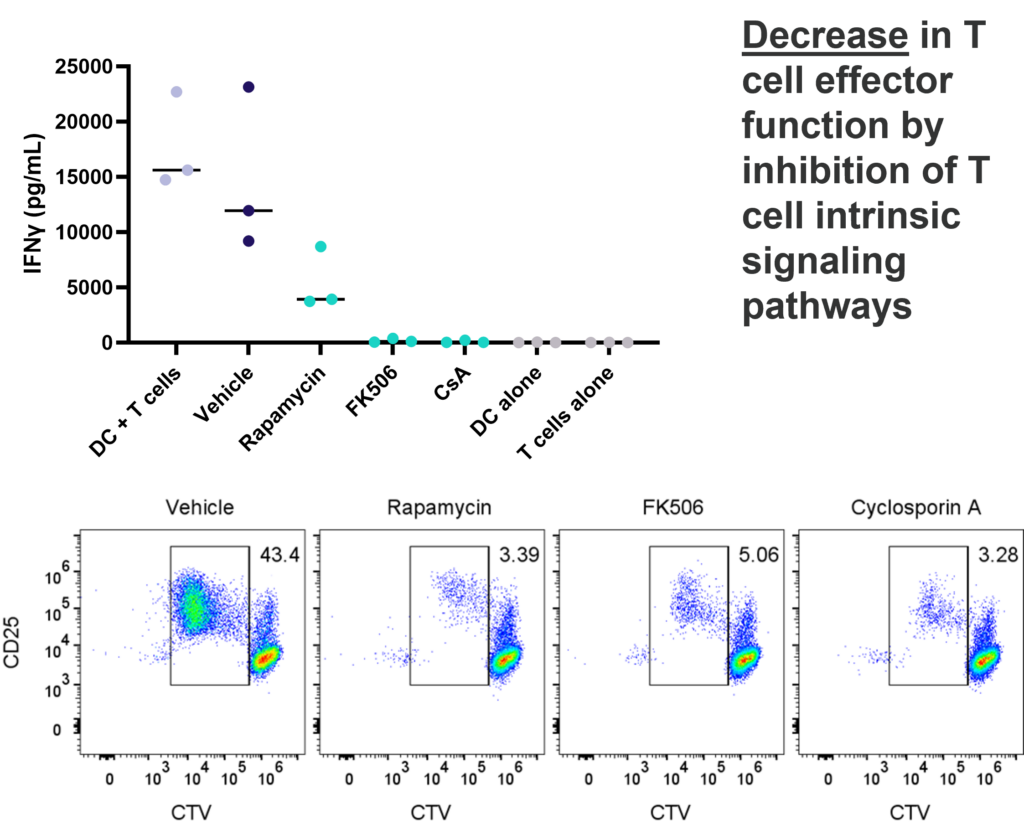
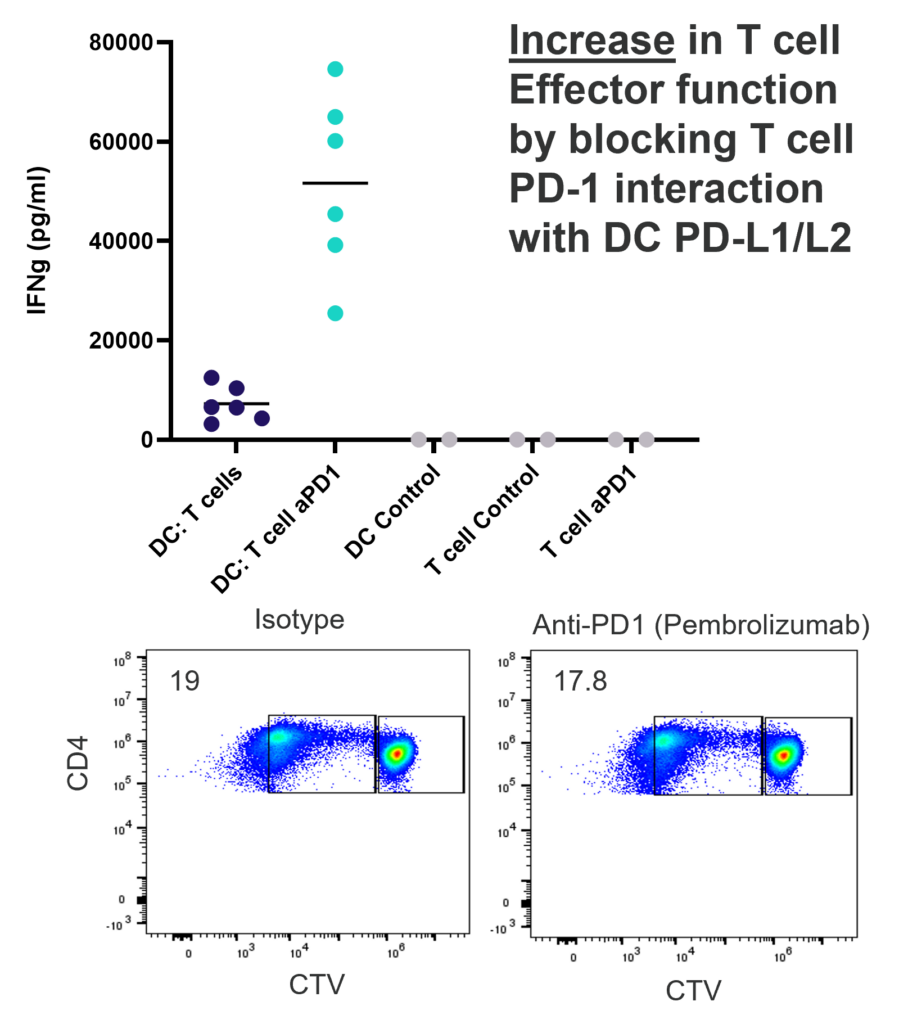
1-way or 2-way MLR. One way MLR of CTV labelled T cells in response to allogenic DC, matured with LPS. T cell proliferation and IFNγ production is inhibited by Rapamycin, FK506 and Cyclosporin A, in contrast, PD-1 blockade (Pembrolizumab) increases T cell IFNγ production, but does not enhance T cell proliferation.
This assay provides a relevant human system to test novel therapeutics that block or engage key co-stimulatory or inhibitory ligands expressed on immune cells.
Evaluation of therapeutic modulation of antigen-specific memory T cell responses to recall antigens
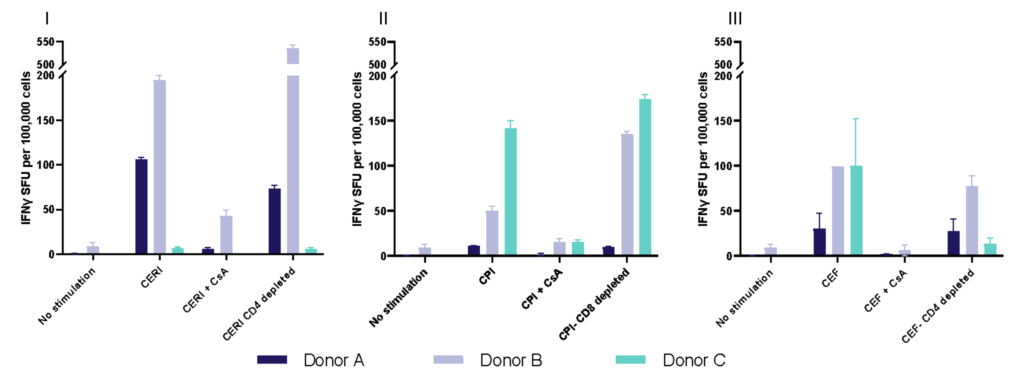
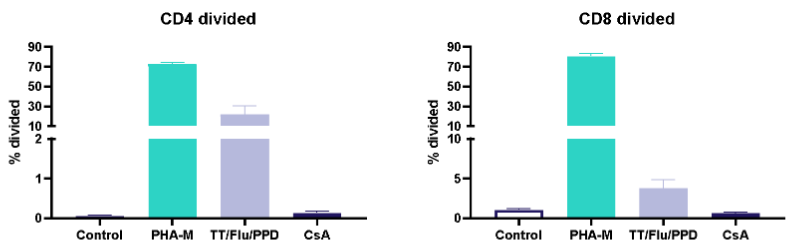
I. T cell response to Tetanus Toxoid, Influenza and PPD antigens
II. Dose response to Influenza antigen
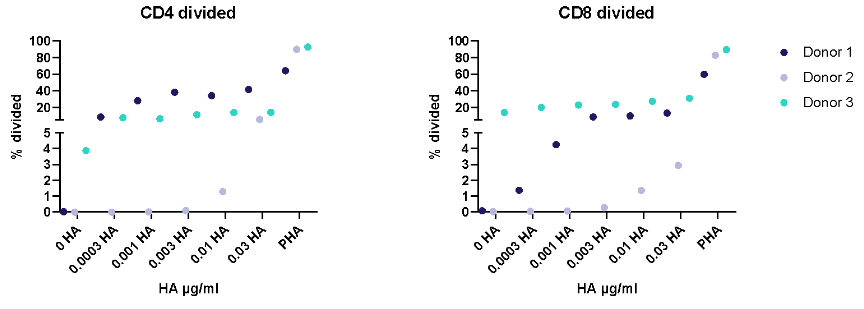
Antigen specific T cells response to a recall antigens. (I) Healthy donors PBMC were stimulated with PHA-M or triple antigen cocktail (Tetanus Toxoid, Influenza and PPD). Cyclosporin was used as a reference treatment. (II) Dose response to Influenza antigen. CD4 and CD8 T cells proliferation was measured by flow cytometry using CTV dilutions.
Therapeutic targeting of CD8+ T cells for Immuno-Oncology and Autoimmunity
3D immune mediated Tumour Killing Models: Tools for screening immune or tumour targeted therapeutics
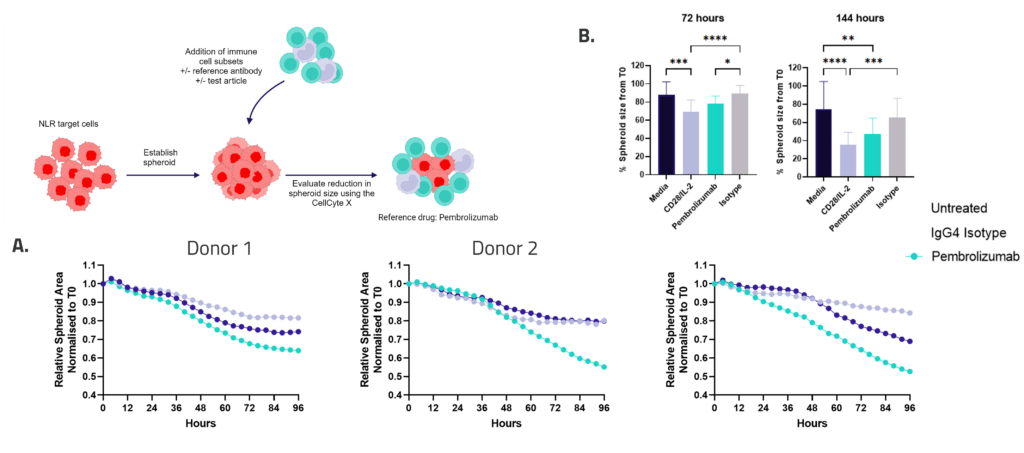
Figure 1: SKOV-3 NLR tumour cells were seeded into 96 well plates, PBMC were added once spheroids were established in the presence of Pembrolizumab or IgG4 control or vehicle (untreated) and imaged every 4 hours for 96 hours using the CellCyte X. Relative spheroid area (%) was measured (A). AUC statistics were calculated using GraphPad Prism v9.5.0
Enzyme-Linked Immuno Spot (ELISpot) enables quantification of rare immune cells in response to antigenic stimulation
ELISpot is a highly sensitive method to test immune modulators, novel vaccine candidates or de-risk immunogenicity testing in an antigen-specific CD4 and/or CD8 T cell assay.
| Condition | Aim |
| No Stimulation | Negative control |
| PMA | Positive control |
| CERI (CMV, EBV, RSV, Influenza) | MHC-I restricted peptide pool to evaluate modulation of CD8+ T-cell memory response |
| CPI (CMV, Parainfluenza, Influenza) | Positive protein antigens to evaluate modulation of CD4+ T-cell memory response |
| CEF (CMV, EBV, Influenza) | MHC-I restricted peptide pool to evaluate modulation of CD8+ T-cell memory response |
| Cyclosporin A (CsA) | Inhibition of immune response |

Spot Forming Units (SPU) for IFN-γ per 100,000 PBMC from CERI, CPI and CEF antigens for three donors

Evaluation of therapeutic modulation of antigen-specific memory T cell responses to recall antigens
I. T cell response to Tetanus Toxoid, Influenza and PPD antigens

II. Dose response to Influenza antigen

Antigen specific T cells response to a recall antigens. (I) Healthy donors PBMC were stimulated with PHA-M or triple antigen cocktail (Tetanus Toxoid, Influenza and PPD). Cyclosporin was used as a reference treatment. (II) Dose response to Influenza antigen. CD4 and CD8 T cells proliferation was measured by flow cytometry using CTV dilutions.
Dendritic cells fed with tumour cell lysates prime rare neoantigen-specific CD4 T cells and cross present antigen to prime neoantigen-specific naive CD8 T cells
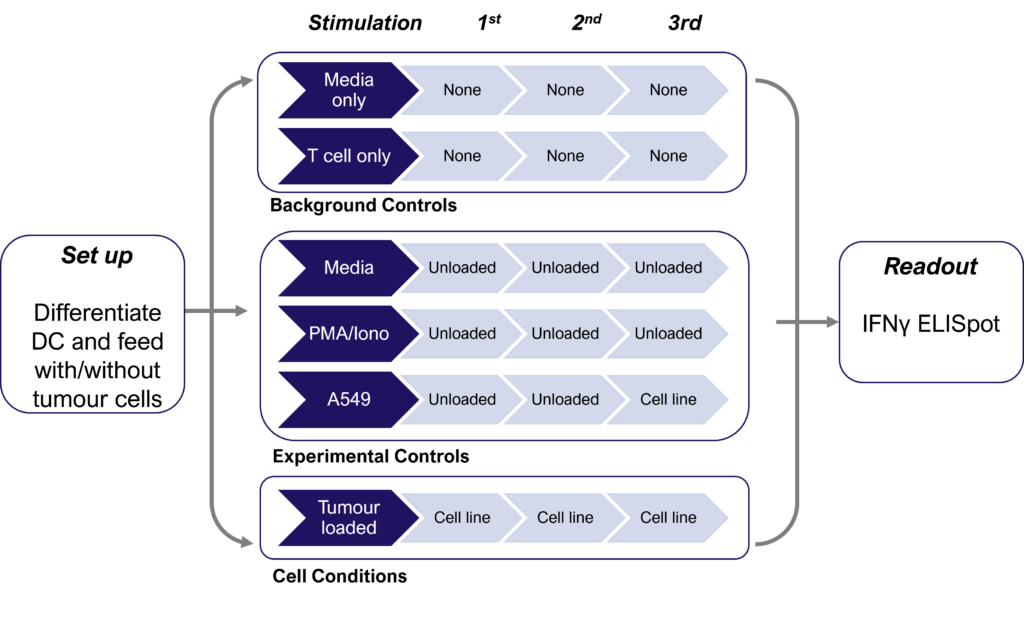
Therapies targeting the modification of tumor cells to increase neoantigen expression and improve immune cell recognition are currently under development. Detecting these subtle changes typically involves several cycles of dendritic cell restimulation of T cells, followed by the sensitive measurement of rare neoantigen-specific T cell responses using IFNγ ELISPOT assays. The schematic below shows the assay design with multiple round of dendritic cell (DC) restimulation to expand rare neoantigen T cell responses.
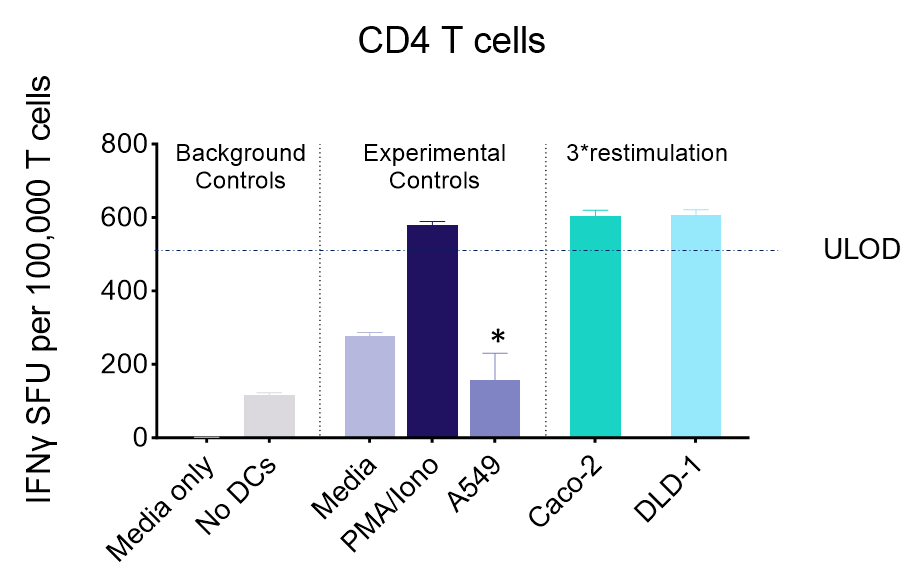
IFNγ release from activated tumour neoantigen-specific CD4 T cells
IFNγ release from activated tumour neoantigen-specific naive CD8 T cells
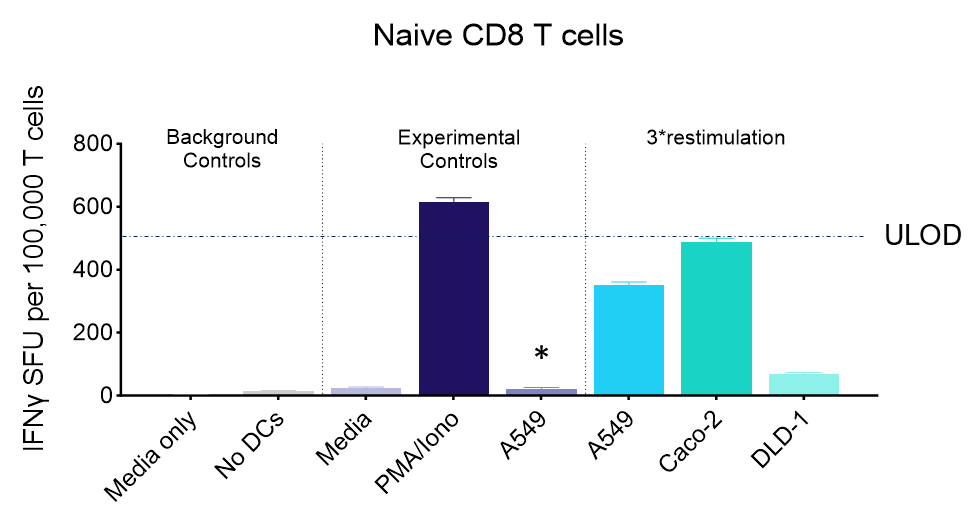
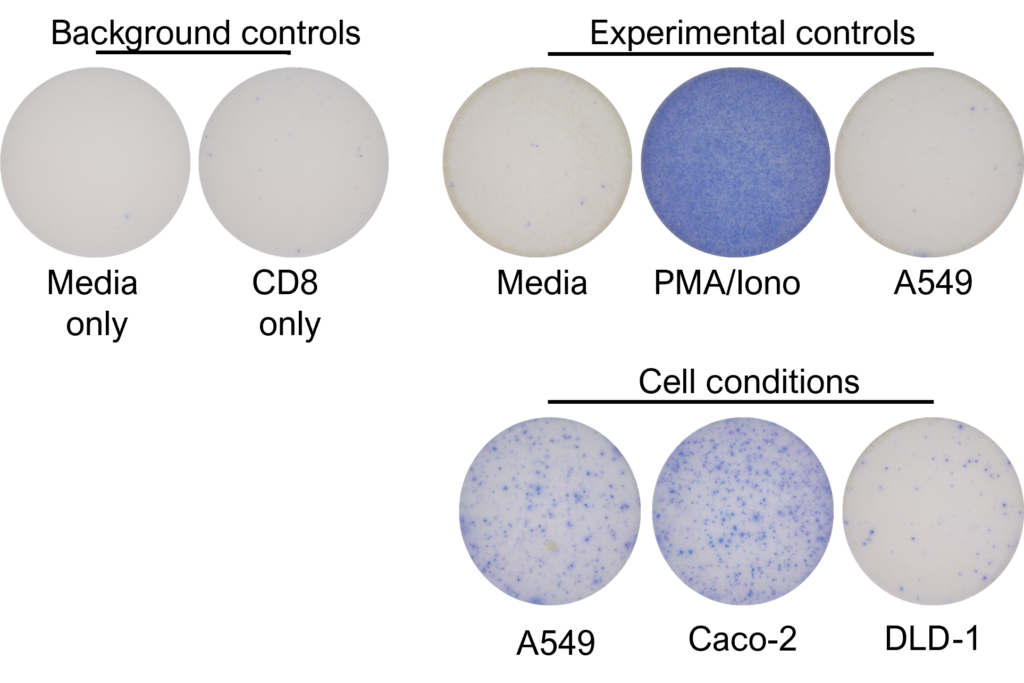
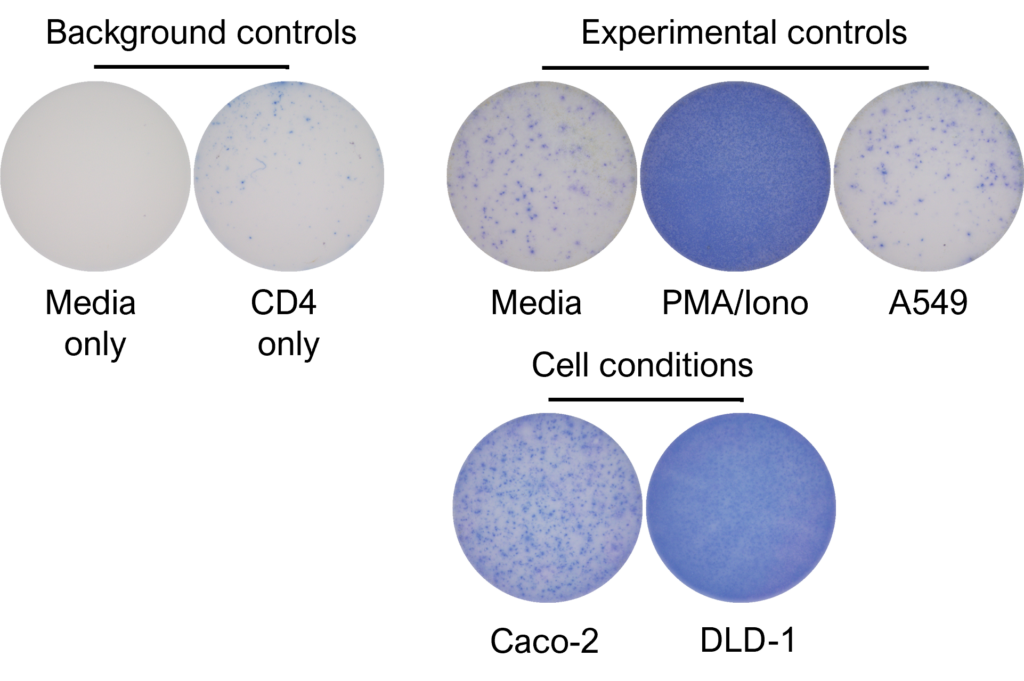
Figures: CD4 or naïve CD8 T cells were co-cultured with unloaded dendritic cells (DC), or with DC loaded with tumour cells that had been previously treated with media or vehicle. Three rounds of stimulation of T cells with DC were performed (* single round of DC stimulation). PMA and ionomycin were added as positive controls during the final round of stimulation. T cell stimulation was assessed by measuring the number of spot forming units (SFU) by IFNγ ELISpot. Media and T cell only wells were included as background controls. Number of SFU per 100,000 plated T cells, with mean of two (experimental controls) or three (all others) replicates ± SEM shown. Representative images of CD4 and naïve CD8 T cell IFNγELISpot. Each blue dot represents one IFNγ-producing T cell. ULOD = Upper Limit of Detection
Tonsillar re-aggregate model
Tonsils are a secondary lymphoid tissue rich in B cell subsets and the specialized, anatomically restricted T follicular helper (Tfh) subset of CD4 T cells. Tonsils provide an accessible source of cells for those interested in B cell immunology, Tfh biology and vaccine development. The tonsillar cell re-aggregate model provides functional insight into human germinal center biology, allowing measurement of key features including naive B cell differentiation, plasma cell/plasmablast differentiation, and antibody production.
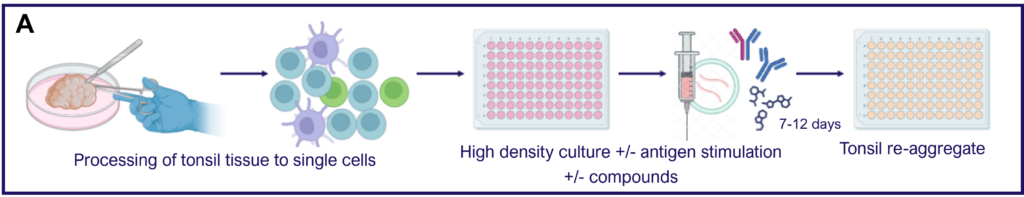
Figure A. Schematic representation of tonsil re-aggregate model. Cells are seeded at high density in 96 well plates; unstimulated (US) or stimulated with quadrivalent inactivated influenza vaccine (IIV) in the presence of vehicle or reference inhibitors such as Rapamycin. B cell readouts include B cell phenotyping, B cell proliferation and hemagglutinin (HA)-specific IgG by ELISPOT or ELISA.
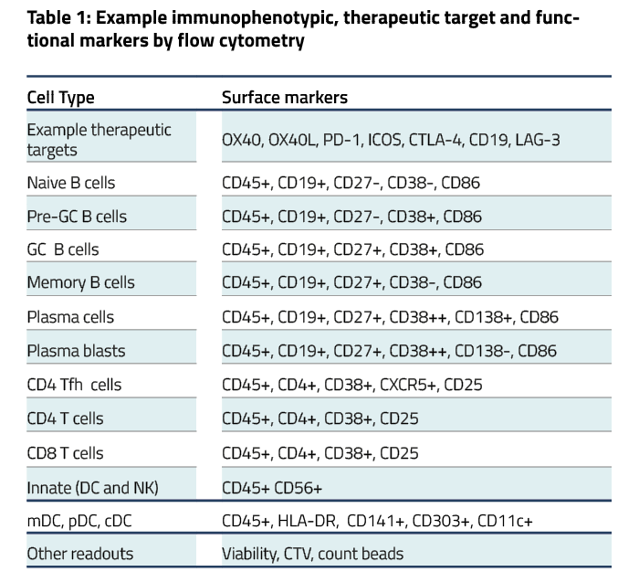
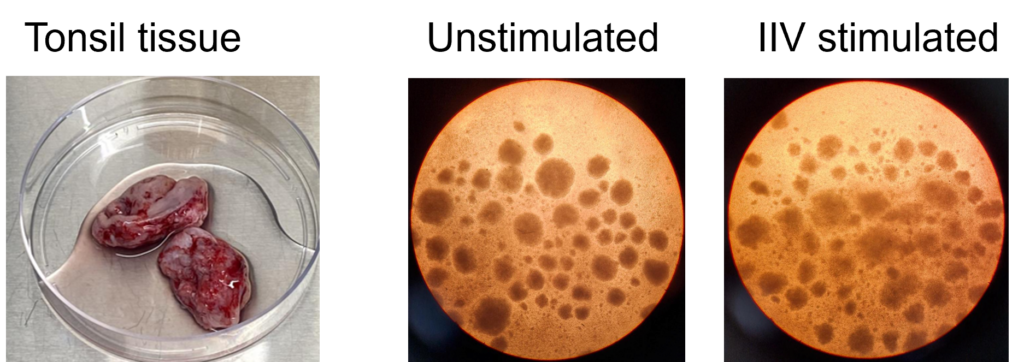
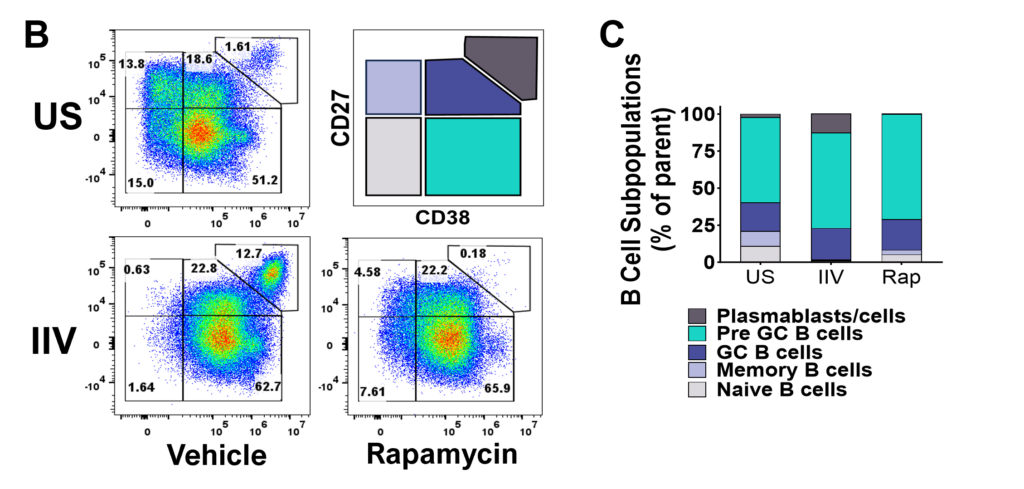
Figure B. Flow plots representing B cell subpopulations. C. Bar graphs showing frequencies of each B cell subpopulation.
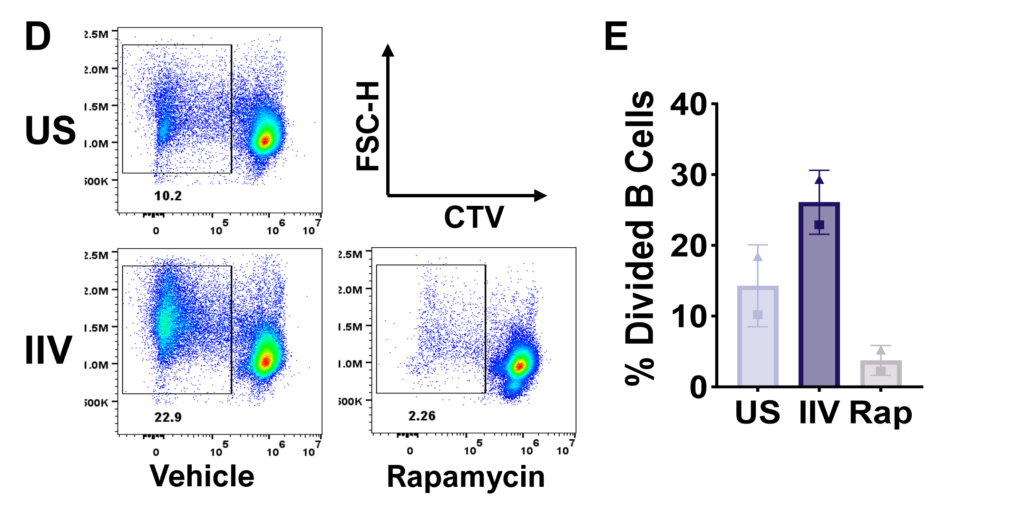
Figure D. Flow plots representing total B cell proliferation. E. Bar graphs showing frequencies of divided B cells. Gating strategy: CD45+ > Viable > FSC vs SSC > Singlets > CD4-CD8- > CD19hi/lo
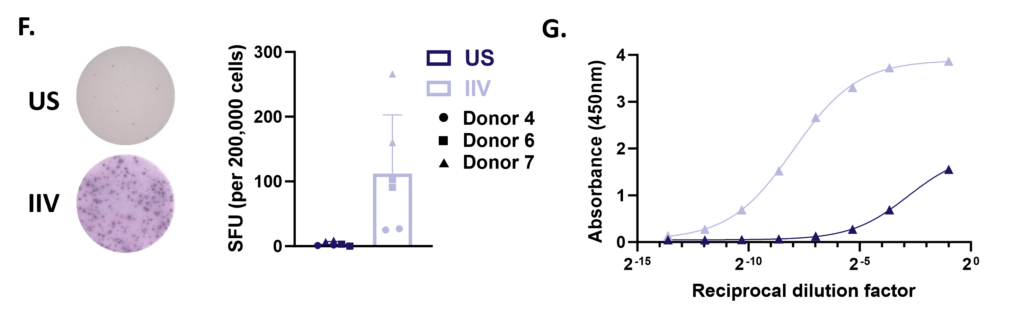
B cell activation, proliferation and Ig secretion
Peripheral blood B cells can be stimulated with T-independent and T-dependent stimuli driving effector functions such as B cell activation, proliferation, Ig production and Ig class switching. Therapeutic intervention using pan or B cell suppressants – Rapamycin, Cyclosporin A or BTK inhibitor Ibrutinib respectively modify B cell function. Other B cell readouts such as downstream signaling following BcR cross linking and B cell antigen presentation to T cells can be assessed.
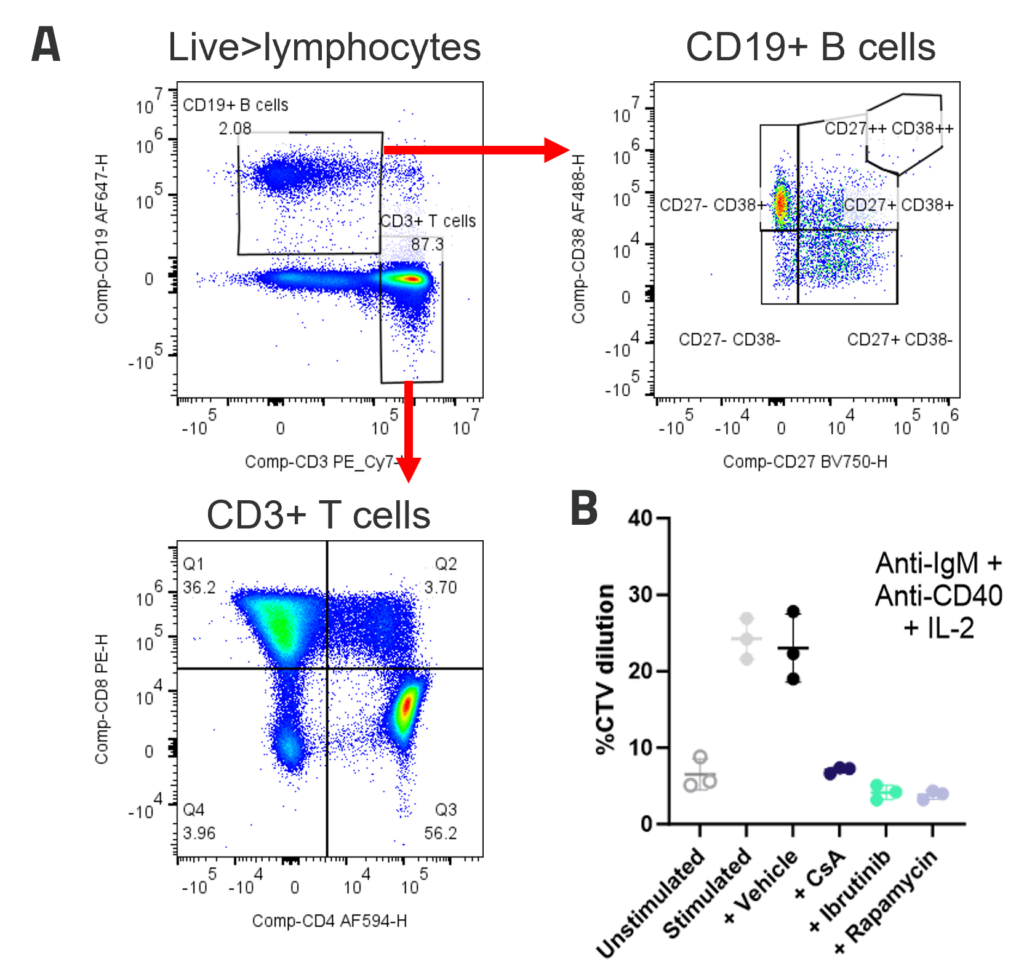
A. Peripheral blood B cells constitute around 2-5% of PBMC and contain predominantly naïve (CD27-CD38+) and memory (CD27+CD38-) populations. Example B and T cell profiles in unstimulated PBMC (B) B cell proliferation to polyclonal T dependent stimuli. PBMC were stimulated with anti-IgM + anti-CD40+ IL-2. Graphs show B cell %CTV dilution as a measure of proliferation Stimulation was performed in the presence or absence of inhibitors; FK506, Ibrutinib or Rapamycin. Bars indicate mean +/- SD, n = 3 donors.
B cell depletion via Antibody-Dependent Cellular Cytotoxicity (ADCC)
B cell depletion is reported to not only have the potential to alter levels of auto-antibodies but also the immune milieu – B cells produce many soluble mediators which impact on other immune cell types such as T cells and DC, so depletion of B cells has the potential to regulate other subsets involved in the disease process. Alemtuzumab was used to show NK cell mediated B cell depletion further examples using Rituximab are available.
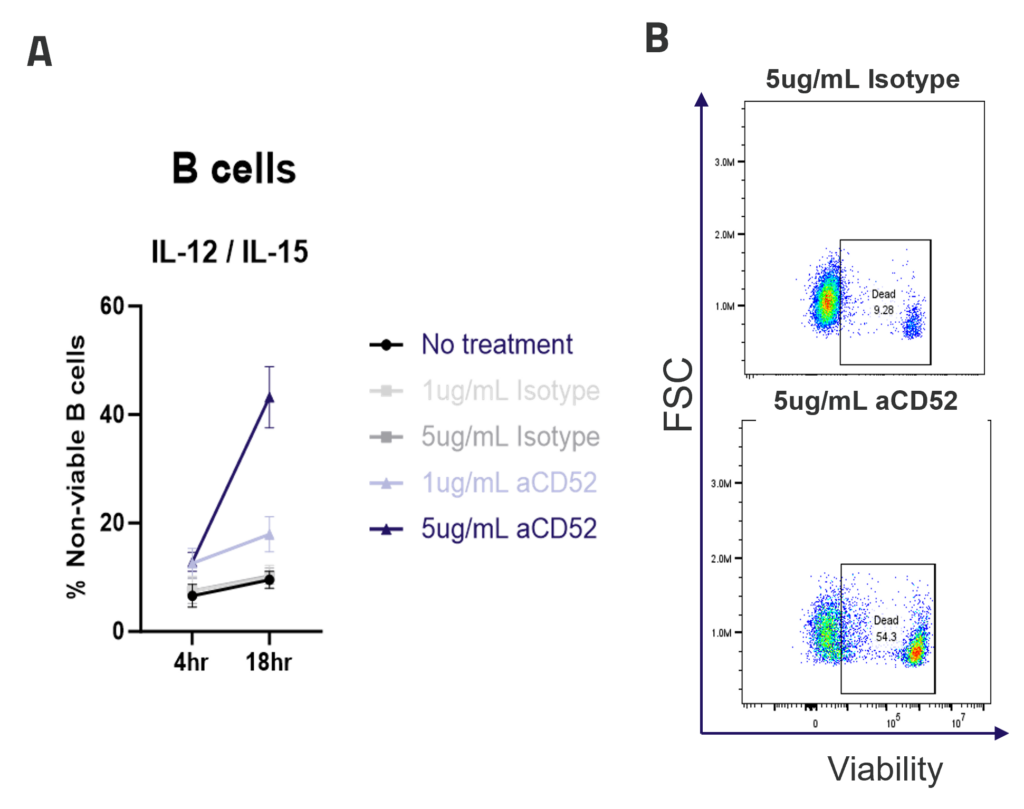
A. ADCC mediated B cell depletion. PBMCs were rested overnight with IL-12/IL-15 pre-activation, before addition of IgG1 isotype control or anti-CD52 Alemtuzumab (1ug/mL, 5ug/mL). The viability of CD4, CD8 and B cells (A) was measured by flow cytometry after 4 or 18hr of culture. Graphs show mean +/- SEM, n = 3 donors. Representative dot plots (B) show B cell viability after 18hr of culture.
